Computational Modeling of Nanoparticle Heating for Treatment Planning of Plasmonic Photothermal Therapy in Pancreatic Cancer
Pancreatic cancer is one of the deadliest cancers, with a 7% survival rate at five years from diagnosis. The limited number of treatment options for patients who are diagnosed with late-stage disease is a major contributor to this problem. Therapeutic modalities such as radiofrequency ablation (RFA) and laser interstitial thermal therapy (LITT) have emerged as potential tumor debulking tools. However, the outcome of these tissue treatments in-vivo is difficult to predict due to visualization constraints, causing a reduction in specificity and increasing pancreatitis risk. Gold nanoparticles (GNPs) may improve the efficacy of thermal therapy. Before in-vivo studies are undertaken, we propose a computational model of plasmonic photothermal therapy (PPTT) to study the laser-particle-tissue interactions and determine the optimum parameters for this treatment. This model may also serve as a future treatment planning tool for physicians.
We compared different shapes of nanoparticles (nanorod, nanosphere, nanobipyramid) and studied the temperature gradient induced in the surrounding media in both water and tissue. The particles were modeled using SOLIDWORKS® CAD software and imported into COMSOL Multiphysics® simulation software via LiveLink™. Tissue properties were extracted from literature. The photothermal behavior of the GNPs when illuminated by a 20 mW, 808 nm laser was described by: (1) using the RF Module to find the amount of energy absorbed, and (2) using the Coefficient Form PDE interface to solve the heat equation using the previously calculated energy absorbed as the heat source and find the temperature gradient surrounding each particle.
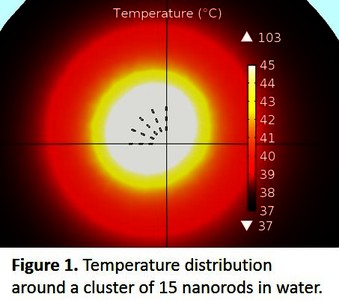
The preliminary results identify the nanorod as the most promising shape for effective implementation of PPTT with our simulations predicting a temperature gradient of at least 8°C within a region of 1 um around a cluster of 15 nanorods in water, as shown in Figure 1. Further simulations on the effects of PPTT in tissue (e.g. including the thermal properties of tissue accounting for laser light source effects) have also been carried out.
Download
- manrique-bedoya_poster.pdf - 1.09MB
- manrique-bedoya_paper.pdf - 1.1MB
- manrique-bedoya_electromed_presentation.pdf - 1.54MB
