Beyond the limits of mass transfer: How pillar electrodes influence electrochemical reactors
Electrochemistry is inherent a surface process, requiring the transport of reagents towards the electrode surface. In electrochemical reactors this typically results in mass transfer limitation problems, limiting the yield and efficiency of the reactor. While the academic community is developing ever more active electrocatalysts, mass transfer towards the electrode surface remains underdeveloped. With the rise of additive manufacturing technologies such as 3D printing, we demonstrated that structured 3D electrodes can result in significant efficiency gains compared to flat electrodes. Nonetheless, insight in optimal electrode designs still lacks.
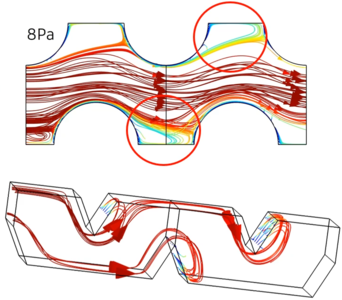
In this work we used COMSOL® simulations to gain insight in the mass transfer performance of different pillar electrodes. Through the use of the Batteries & Fuel Cells Module, the stationary laminar flow and concentration profile was computed for each pillar electrode design, allowing to derive volumetric mass transfer coefficients as function of the applied hydrodynamic parameters. From these results it was observed that the mass transfer coefficient strongly varied with the pillar shape, interpillar distance and pillar size. Changing the pillar shape from cylindrical pillars to diamond shaped ones resulted in a two-fold increase of the mass transfer properties and revealed significant mixing effects at critical operating conditions (i.e. fluid velocity and pressure drop). Decreasing the interpillar distance resulted up to a 50 fold increase in mass transfer properties without an increased pressure drop or pumping cost. Simulations at varying pillar sizes demonstrated an optimum in pillar size exist, yielding maximal mass transfer properties. It is clear that through computational fluid dynamics simulations necessary insights in the electrode design can be obtained, opening the door to improved electrode and electrochemical reactor designs.