Modeling Galvanic Corrosion
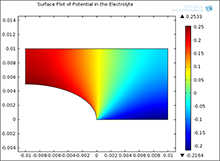
Galvanic corrosion is encountered in marine applications because one often has dissimilar metal joints and seawater acts as an electrolyte. One metal acts predominantly as anode and undergoes material dissolution while the other acts predominantly as cathode and is the site where a cathodic reaction takes place. Assuming a stagnant electrolyte, the equation governing the distribution of electrochemical potential is Laplace’s equation. The equation must then be solved subject to Butler-Volmer type boundary conditions. The coefficient form PDE study in COMSOL Multiphysics® has been used to develop a broad variety of models of galvanic corrosion systems. Results are in excellent agreement with results previously obtained for selected test problems.
Download
- gutierrezmiravete_presentation.pdf - 1.17MB
- gutierrezmiravete_paper.pdf - 0.42MB
- gutierrezmiravete_abstract.pdf - 0.08MB
